main content start
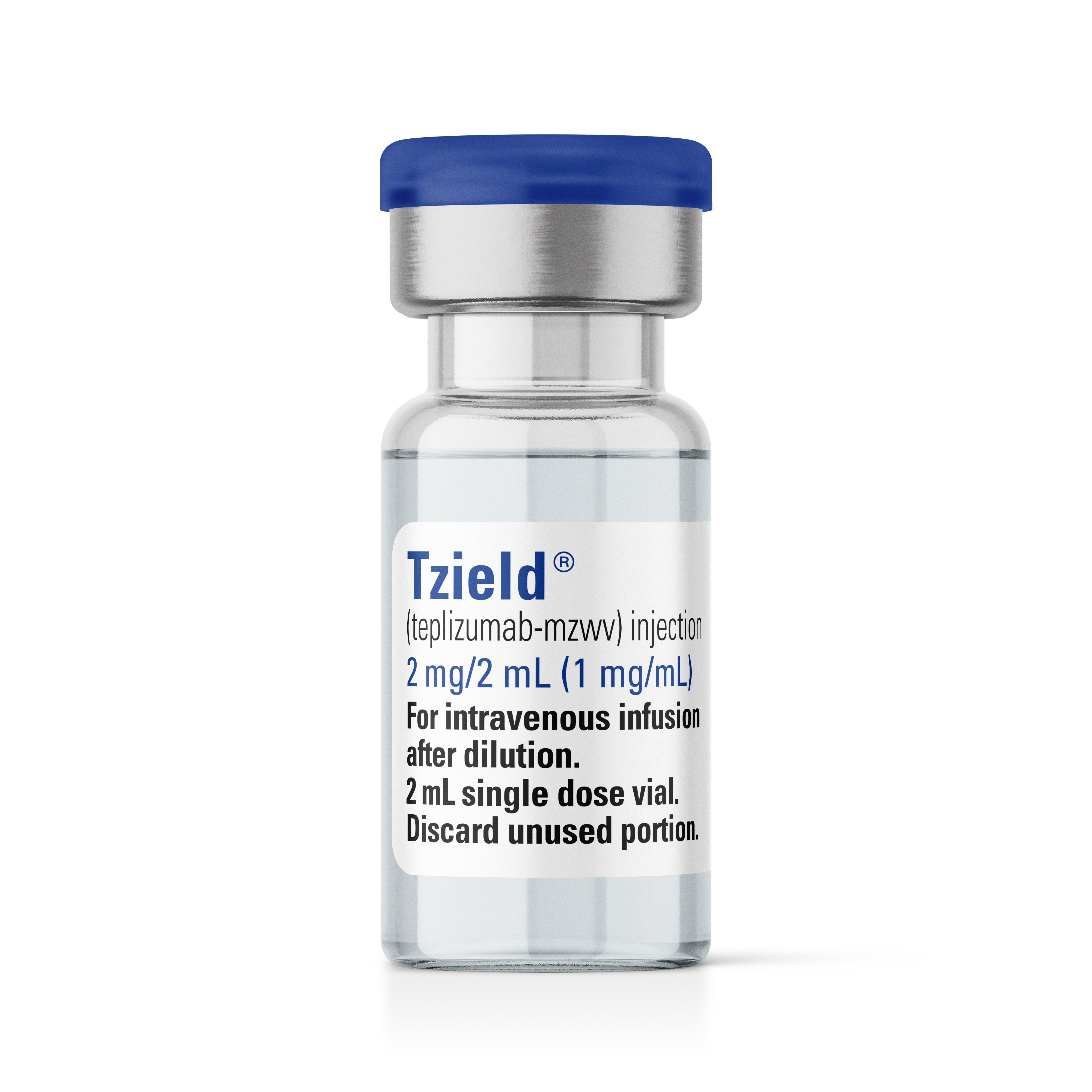
Tzield is a CD3 antibody approved to delay the onset of stage 3 type 1 diabetes in people over the age of 8. The medication is given to people with stage 2 type 1 diabetes, meaning they have two or more pancreatic islet autoantibodies damaging the pancreatic beta cells that make insulin, leading to disrupted glucose management. Tzield is given via infusion daily over 14 consecutive days. After infusion, Teplizumab-mzwv binds to certain immune system cells and is thought to work by stopping the immune system attack on the insulin-producing beta cells in the pancreas.
- Tzield binds CD3 on T cells, reducing cytotoxicity and promoting regulatory T cells to preserve beta cell function, delaying progression to stage 3 type 1 diabetes
- FDA-approved to delay stage 3 type 1 diabetes in patients with stage 2, confirmed by ≥2 islet autoantibodies and dysglycemia without type 2 diabetes
- Given as a daily IV infusion over 30 minutes for 14 consecutive days; premedication (antipyretics, antihistamines) mitigates cytokine release syndrome (CRS)
- Common adverse reactions include lymphopenia (73%), rash (36%), leukopenia (21%), and headache (11%); read boxed warnings for CRS and serious infections
Tzield Specs
Key specification
Generic Name
Teplizumab-Mzwv
Approved for Kids
8+ with Stage 2 T1D
Drug Class
Monoclonal antibody
Type
Infusion
General Information
Product Name
Tzield
Product Type
Injectable Medications
Brand
Sanofi
Other Devices & Supplies
Looking for a different way to manage diabetes care? Browse and compare information on the latest devices, tools, and technology.